Science Policy and Government Affairs
In addition to fostering innovative cancer science and medicine, the AACR pursues its mission to prevent and cure all cancers by addressing the policy and regulatory challenges that can impede progress against cancer. The AACR Office of Science Policy and Government Affairs works with lawmakers and federal agency personnel, including regulators, on behalf of the cancer research community, providing expert guidance to maximize the impact of policy decisions on cancer patients.
Continued Support for Medical Research
Under the leadership of its Science Policy and Government Affairs Committee (Chair: George D. Demetri, MD), the AACR worked with the medical research community in 2019 to make the case to legislators for robust, sustained, and predictable annual funding increases for medical research. These efforts yielded results in December, when Congress approved a $2.6 billion FY 2020 budget increase for the NIH—including a $297 million increase for the NCI and a $91 million increase for the FDA. The FY 2020 budget marked the fifth consecutive year of significant funding increases for the NIH, resulting in a 39 percent increase in the agency’s budget since FY 2016.
- Early Career Hill Day. The AACR and its Associate Member Council brought 16 Associate Members to Washington, DC, in February for the fourth annual AACR Early-Career Hill Day. Accompanied by staff from the AACR Office of Science Policy and Government Affairs, these early-career scientists visited the offices of their Senators and congressional representatives to urge them to vote for continued robust funding for the NIH and NCI.
- AACR-AACI Hill Day. The AACR and the Association of American Cancer Institutes (AACI) brought together nearly 100 advocates from 24 states—including cancer center directors, scientists, clinicians, and survivors—to Washington, DC, in April for their annual Hill Day. The participants visited the offices of their congressional representatives to emphasize the critical impact that biomedical research funding has on the lives of cancer patients.

- AACR Cancer Progress Report Briefing. At a congressional briefing in September, the AACR presented the ninth annual AACR Cancer Progress Report to members of Congress and their staffers. Hosted by AACR President (2019–2020) Elaine R. Mardis, PhD, FAACR (far right), and Chief Executive Officer Margaret Foti, PhD, MD (hc) (center), the briefing featured cancer survivors and scientists outlining how federally funded research is spurring improvements in public health and innovative breakthroughs across the spectrum of cancer care.
- Rally for Medical Research Hill Day. In September, more than 350 organizations representing patients, caregivers, researchers, and health care professionals participated in the seventh annual Rally for Medical Research Hill Day in Washington, DC. Advocates from 35 states learned about the appropriations process and effective strategies for communicating with lawmakers about the importance of robust annual funding increases for the NIH. Several members of Congress known as champions of biomedical research funding addressed the participants prior to their visits, including House Labor-HHS-Education Appropriations Subcommittee Chairwoman Rosa DeLauro (D-CT), Jamie Raskin (D-MD), Peter King (R-NY), Donna Shalala (D-FL), and ranking member of the Labor-HHS-Education Appropriations Subcommittee Tom Cole (R-MO).
- Advocacy from Fellows of the AACR Academy. In December, during a strategically important time on Capitol Hill when Congress was in the early stages of completing the FY 2020 appropriations process, 88 Fellows of the AACR Academy sent a letter to House and Senate appropriations leaders. The Fellows thanked the leaders for their past support of biomedical research and urged them to provide a robust funding increase for the NIH and NCI.

Tobacco Products and Cancer Subcommittee:
Roy S. Herbst, MD, PhD, Chair
The mission of the AACR Tobacco Products and Cancer Subcommittee is to foster scientific and policy initiatives to reduce the incidence of disease and mortality due to tobacco use. In 2019, the subcommittee provided expert comments to the FDA on proposed regulations to reduce the use of e-cigarettes by youth and young adults, to designate tobacco use in electronic health records, and to require graphic warnings for cigarette packages and advertisements.
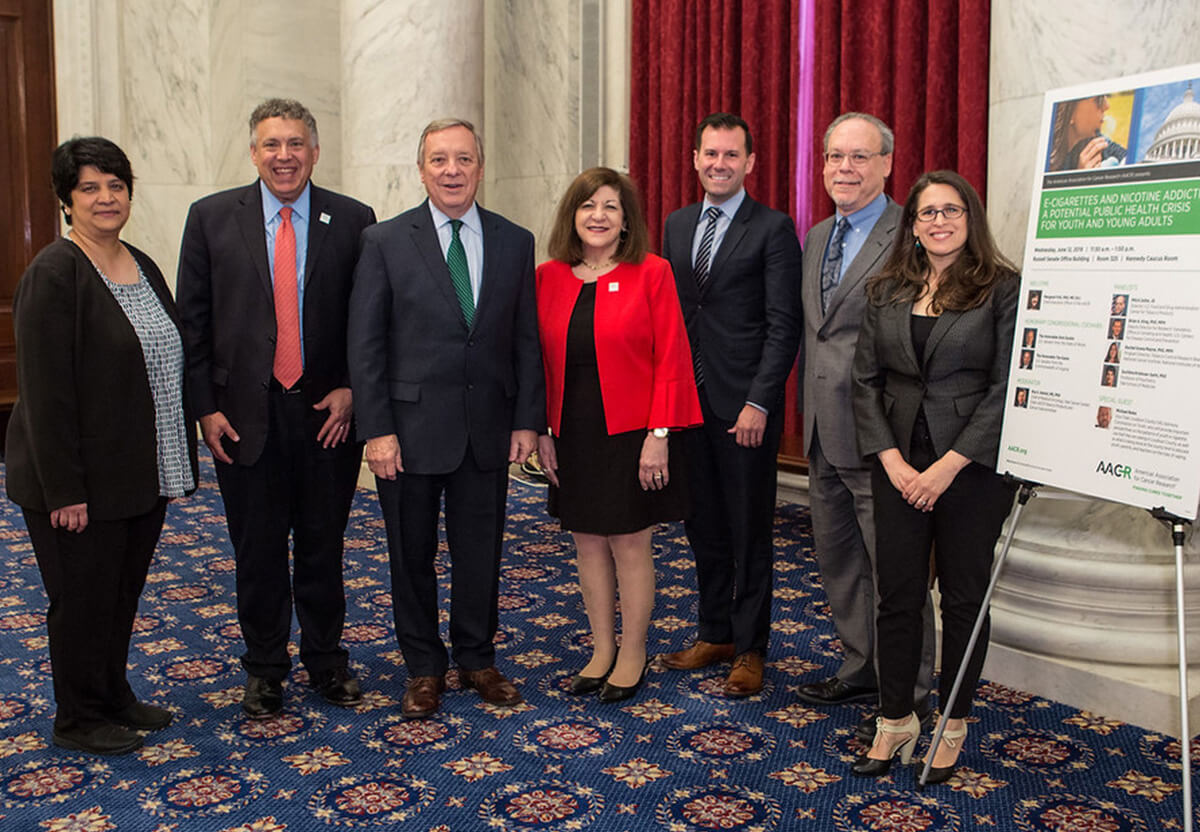
In June, the subcommittee also hosted a congressional briefing on “E-cigarettes and Nicotine Addiction: A Potential Public Health Crisis for Youth and Young Adults.” Cosponsored by Senators Dick Durbin (D-IL) and Tim Kaine (D-VA), the briefing included subject matter experts from the FDA, NCI, CDC and Yale Cancer Center who addressed the dangers of increased e-cigarette use among youth and young adults in the United States.
Health Policy Subcommittee:
Gilbert S. Omenn, MD, PhD, Chair
The AACR Health Policy Subcommittee promotes policies and develops educational initiatives that foster the closer integration of clinical practice and cancer research. In June, the subcommittee collaborated with the Moffitt Cancer Center and the Biden Cancer Initiative to co-host a congressional briefing titled “Let’s End HPV-Related Cancers.” The briefing— was sponsored by Rep. Kathy Castor (D-FL)—was also developed in partnership with the American Cancer Society Cancer Action Network, American Society of Clinical Oncology, Association of American Cancer Institutes, Prevent Cancer Foundation, St. Jude Children’s Research Hospital, and the Union for International Cancer Control. It discussed the latest interventions against HPV-related cancers and outlined a path toward elimination of these cancers.
Regulatory Science and Policy Subcommittee: Kenneth C. Anderson, MD, FAACR, Chair
The AACR Regulatory Science and Policy Subcommittee develops and implements programs and policy initiatives to improve the development, evaluation, and regulation of cancer drugs, biologics, and devices. The subcommittee worked with stakeholders in academia, industry, advocacy, and government to host two critical meetings in 2019:
- FDA-AACR Real-World Evidence Workshop. In July, the AACR and the FDA cosponsored this workshop to discuss the implications and potential for real-world evidence (RWE) in oncology. Chaired by Pallavi Mishra-Kalyani, PhD, and Sean Khozin, MD, MPH, from the FDA and AACR representative Deborah Schrag, MD, MPH, the workshop focused on practical use cases that described recent efforts to use medical claims data, electronic health records, patient-reported outcomes, and product or disease registry data to provide critical clinical insights.
- NIH-AACR Cancer, Autoimmunity, and Immunology Conference. In April, the AACR and NIH organized a meeting of preeminent researchers on the NIH campus to discuss adverse events resulting from the treatment of cancer with immunotherapies. Featuring keynote addresses from AACR President (2018–2019) Elizabeth M. Jaffee, MD, FAACR, Arlene H. Sharpe, MD, PhD, and Jennifer A. Wargo, MD, MMSc, the conference explored the biology of immune-related adverse events and how the study of autoimmune disease could improve the treatment of these events.

AACR Annual Meeting: Science Policy and Regulatory Science and Policy Tracks
Recognizing the critical need to educate cancer researchers and other health care professionals about science policy and regulatory issues, the AACR developed an expanded track of 16 science policy and regulatory science and policy sessions at the AACR Annual Meeting in April. The track covered a wide range of topics, including the use of science to inform e-cigarette policy, the impact of Brexit on oncology drug development and regulation, and the impact of China’s expanding role as both a developer of and a market for cancer pharmaceuticals. Among the most popular sessions was “PD-1 Pandemonium: FDA Speaks with Industry on the Past, Present, and Future of PD-1 Drugs.” In the session, Oncology Center of Excellence Director Richard Pazdur, MD, moderated a panel discussion with industry experts that addressed the challenges and opportunities pertaining to the development of PD-1/PD-L1 immunotherapeutics.
Science Policy Fellowship
In addition to sustaining the cancer workforce through science education and career development, the AACR took steps in 2019 to build the science policy and advocacy workforce with the launch of its Science Policy Fellows program. The program enables early-career cancer researchers—like J. Tod Guidry, PhD, the inaugural policy fellow—to work on significant science and health policy issues, key regulatory science and policy topics, and important matters of interest to those on Capitol Hill, while working in various offices and agencies in Washington, DC.
Survivor and Patient Advocacy
Survivor and Patient Advocacy
The AACR’s scientific programs and initiatives bring together scientists, clinicians, and other health care professionals and focus their efforts on improving the lives of cancer patients. Through its Survivor and Patient Advocacy Program, the AACR also brings patients into the community of cancer professionals, educating them about the science behind cancer research and treatment and empowering them to inform that process by sharing their perspectives.
Scientist↔Survivor Program
The AACR Scientist↔Survivor Program (SSP) builds enduring partnerships among leaders of the scientific, survivor, and patient advocacy communities by bringing them together at AACR scientific meetings to share the latest innovative cancer science. Survivors and advocates attend focused lectures and scientific sessions with scientist mentors, fostering an exchange in which patients and advocates learn about the biology behind treatment decisions and scientists better understand the impact their work has on the patient experience. More than 60 patient advocates participated in the program at the AACR Annual Meeting 2019 in Atlanta and at the Twelfth AACR Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved in San Francisco.
HIGHLIGHTING SURVIVORSHIP AND ADVOCACY
As part of the dialogue between cancer professionals and patients, the Survivor and Patient Advocacy program worked with meeting organizers to place patient- and advocate-focused sessions on the programs of three AACR meetings in 2019:
- AACR Annual Meeting 2019. The Annual Meeting program included a complete Science of Survivorship Track consisting of 12 sessions on topics such as data sharing, survivorship and aging, and involving patients in the research process. The track included special poster sessions in which Scientist↔Survivor Program participants discussed their advocacy initiatives and research.
- Science of Cancer Health Disparities Conference. The program for this conference included a special session titled “Addressing Advocacy at the Bench: Implementing Change.” Recognizing that a contributing factor to health disparities is the lack of participation in research studies by underserved populations, the session offered examples of community-based initiatives to increase patient engagement in the research process.
- San Antonio Breast Cancer Symposium. The AACR worked with the Alamo Breast Cancer Foundation to present a special education session for cancer advocates and health professionals. Julie R. Gralow, PhD, professor of breast medical oncology and professor of global health at the University of Washington School of Medicine, discussed "New Strategies in the Treatment of Breast Cancer."

Cancer Today Magazine
Cancer Today—the AACR’s magazine and website for cancer patients, survivors, and caregivers—is a vital resource for anyone navigating the challenges of cancer diagnosis, treatment, and survival. Now in its eighth full year of publication, the magazine continues to tackle important cancer topics in a serious, comprehensive way. Among the most read stories published in 2019 were the following:
- “First Immunotherapy Approved for Breast Cancer.” In March 2019, the Food and Drug Administration issued the first U.S. approval for the use of an immunotherapy drug to treat breast cancer.
- “What Not to Say to a Cancer Patient.” Well-meaning family members and friends sometimes say unhelpful things to cancer patients. Cancer survivor Michael Gavaghen offers examples of statements to avoid when talking to cancer patients about their condition.
- “Lynch Syndrome Linked to More Cancers.” Lynch syndrome is an inherited genetic disorder that increases risk for some types of cancer. A study indicates that testing tumors for certain genetic markers may identify people with previously undiagnosed Lynch syndrome.
- “Still in the Game.” ESPN reporter Holly Rowe stays active after a diagnosis of melanoma and several rounds of treatment. Although still busy covering sports, Rowe now also works with several patient advocacy groups.
- “New Rule Would Require Breast Density Disclosure.” A proposed federal rule would mandate that patients be informed after mammograms if they have dense breasts. Having dense breasts is a risk factor for breast cancer and may make cancer harder to spot. But some warn the rule could have unintended consequences.
Distinguished Public Service Awards
The AACR Distinguished Public Service Award honors the extraordinary contributions of an individual or group whose groundbreaking, innovative work exemplifies the organization’s mission. During the opening ceremony of the 2019 Annual Meeting, the AACR honored three individuals for their outstanding efforts to advance cancer science for the benefit of patients.
2019 AACR Distinguished Public Service Awards

Nancy F. Goodman, JD
Founder and Executive Director, Kids v Cancer
Washington, DC
Ms. Goodman founded Kids v Cancer in 2009, after her 10-year-old son, Jacob, died from medulloblastoma. The nonprofit organization’s mission is to promote pediatric cancer research by identifying policy impediments at key junctures in the research process and developing strategies to address them. She received the Distinguished Public Service Award in recognition of her outstanding leadership in cancer science policy and advocacy, including her exceptional stewardship of two significant federal laws that are providing pediatric cancer patients with additional treatment options.

Louis M. Weiner, MD
Director, Georgetown Lombardi Comprehensive Cancer Center
Associate Vice President, Georgetown University Medical Center
Washington, DC
Dr. Weiner has dedicated his research career to developing and optimizing monoclonal antibody–based immunotherapies. As inaugural Chair of both the AACR Cancer Immunology Task Force and the AACR Cancer Immunology Working Group, he worked to establish cancer immunology as a scientific priority for the organization. He received the Distinguished Public Service Award in honor of his extraordinary research career and contributions to establishing the AACR at the forefront of cancer immunology

Daniel D. Von Hoff, MD, FAACR
Physician-in-Chief and Distinguished Professor, Translational Genomics Research Institute
Chief Scientific Officer, Honor Health Research Institute
Professor of Medicine, Mayo Clinic and University of Arizona College of Medicine
Phoenix, Arizona
Dr. Von Hoff’s cutting-edge research has contributed to the development of more than a dozen FDA-approved agents that are routinely used in the treatment of cancer—including the first combination therapy to demonstrate improved response rates, overall survival, and progression-free survival in patients with metastatic pancreatic cancer.
Dr. Von Hoff has also provided visionary leadership to the field of clinical cancer research. Notably, in 1996 he established the AACR-ASCO Methods in Clinical Cancer Research Workshop, which has trained more than 4,000 clinical fellows and junior faculty clinical researchers around the world in the essentials of novel oncology clinical trial designs. He received the Distinguished Public Service Award for his extraordinary contributions to clinical cancer research and the training of early-career clinical investigators.